Oseltamivir
Oseltamivir (C16H28N2O4) sī 1-ê khòng-pēⁿ-to̍k io̍h-á, iōng lâi ī-hông kap tī-liâu liû-hêng-sèng kám-mō· pēⁿ-to̍k A kap B-hêng. Che sī tē-1-ê siang-gia̍p-kài (Gilead Sciences) gián-hoat chhut-lâi ê kháu-ho̍k-sèng sîn-keng-an-sng-mûi at-chè-sò· (neuraminidase inhibitor), bo̍k-chêng iû Hoffman-La Roche (Roche) hêng-siau, chiūⁿ-chhī ê mâi hō-chò Tamiflu®, Tâi-oân hō-chò Khek-liû-kám, mā ū hō-chò Khek-bín-hok.
Oseltamivir sī 1-ê prodrug, tī koaⁿ lāi-té chúi-kái liáu-āu chiâⁿ-chò ū oa̍h-sèng ê tāi-siā-bu̍t (metabolite).
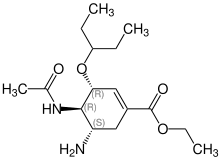

Seng-sán kòe-têng
siu-káiShikimic acid sī oseltamivir seng-sán koè-têng ê tiōng-iàu sêng-hūn, chú-iàu lâi-goân sī peh-kak.
Elias Corey (1990 nî ê Nobel Hòa-ha̍k Chióng ti̍t-chú) kap i ê ha̍k-seng tī 2006 nî hoat-bêng sin khoán ê chè-chō pō͘-sò͘, oân-choân m̄-bián i-lāi peh-kak.[1]
Siong-koan bûn-chiuⁿ
siu-kái- Amantadine, rimantadine - M2 at-chè-sò·.
- zanamivir - lēng-gōa 1-chióng sîn-keng-an-sng-mûi at-chè-sò·.
- Khîm-liû-kám
- 2009 nî liû-kám toā-liû-hêng
Chham-khó chu-liāu
siu-kái- ↑ Yeung, Y.-Y., Hong, S., kap Corey, E.J. "A Short Enantioselective Pathway for the Synthesis of the Anti-Influenza Neuramidase Inhibitor Oseltamivir from 1,3-Butadiene and Acrylic Acid". J. Am. Chem. Soc., 2006, tī 2006 nî 5 goe̍h 7 chham-khó http://pubs.acs.org/cgi-bin/sample.cgi/jacsat/asap/html/ja0616433.html
Gōa-pō· liân-kiat
siu-kái- Tamiflu koaⁿ-hong chām
- Siāng sin ê Oseltamivir gián-kiù Archived 2006-05-07 at the Wayback Machine.
- MedlinePlus i-io̍h chu-sìn
- Bí-kok FDA ê chu-sìn
| Pún bûn-chiuⁿ sī chi̍t phiⁿ phí-á-kiáⁿ. Lí thang tàu khok-chhiong lâi pang-chō͘ Wikipedia. |